Indonesia may soon get a new, much more affordable COVID-19 screening method developed by researchers in the country.
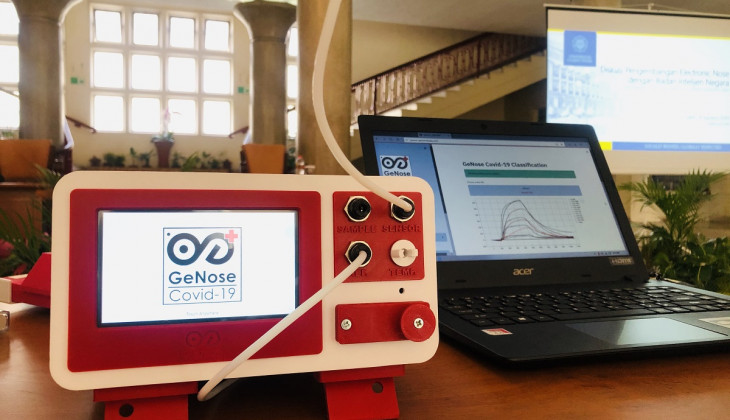
The Health Ministry has approved the distribution of a coronavirus breathalyzer, named GeNose, which was developed and trialed by Yogyakarta’s Gadjah Mada University (UGM).
Researchers at the university say the device offers a pain-free test experience as it would only require that subjects blow into a tube, which would then be analyzed for volatile organic compounds related to the coronavirus that causes COVID-19 with an accuracy rate of 93 percent.
A test would only cost around IDR15K-25K (US$1.06-1.76) and the result can be available in just two minutes.
“With a batch of 100 devices we will soon distribute [to hospitals and labs], we hope we will be able to do 120 tests per device, or 12,000 tests per day. The 120 estimate is based on the three minutes required to test each subject, which includes [blowing into the device], so in one hour the device can test 20 people if it functions for six hours,” lead researcher Kuwat Triyana said in a press release.
Kuwat also said plans are in motion to mass produce GeNose, with the expectation that 10,000 devices can be distributed nationwide by the end of February. Should this target be achieved, Indonesia should be able to screen 1.2 million people for the coronavirus daily.
The Indonesian Society of Respirology (PDPI) praised GeNose’s streamlined testing process, but said that, for the time being, it should only be used as a preliminary screener while PCR swab tests must remain the gold standard for COVID-19 diagnosis per WHO protocol.
In Indonesia, a standard PCR swab test is available for around IDR900K (US$63.50) with the result usually available in two days. Same day test results are available at around double the standard price.




Reader Interactions