Field testing of the COVID-19 tests developed by scientists from the University of the Philippines is expected to end on April 1, according to the Department of Science and Technology (DOST), which funded the research.
DOST Secretary Fortunato dela Peña said today in a Facebook post that the Food and Drug Administration (FDA) is expected to issue a certificate of product registration on the locally made kits on April 3. The kits were approved by the government earlier this month, but weren’t yet ready for commercial use because they still had to be validated in compliance with FDA rules.
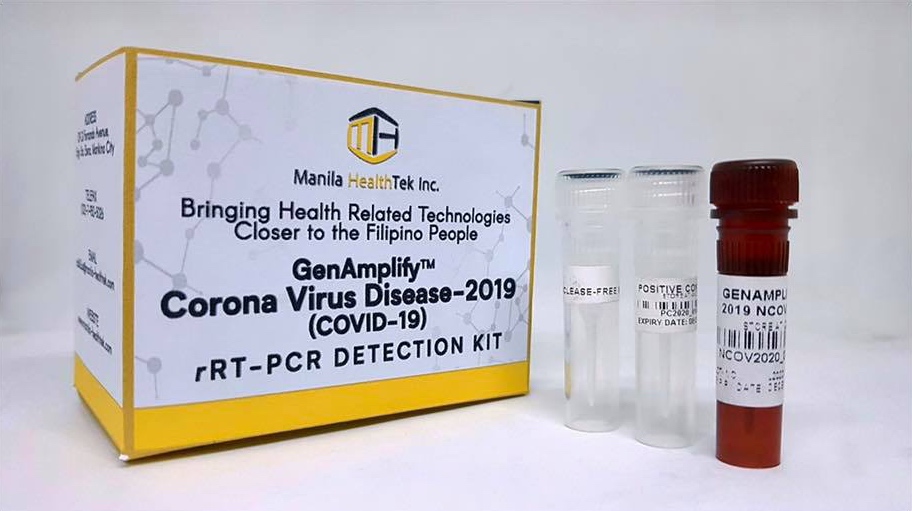
Dela Peña said that the kits’ manufacturer, Manila HealthTek Inc., is ready to produce up to 120,000 units once the FDA has approved their release.
The DOST chief said that some 26,000 tests will be distributed to several major hospitals, including designated COVID-19 facility Philippine General Hospital, as well as the Makati Medical Center and Baguio General Hospital. The remaining 94,000 kits will be sold commercially by Manila HealthTek at around PHP1,300 (US$25) per kit, or at roughly one-sixth the cost of units currently being used in hospitals.
The manufacturers have “enough orders from the private sector who intend to donate them in turn to the Department of Health and hospitals,” Dela Peña said.
In a separate announcement, the FDA said that it has approved five rapid test kits, which were reportedly “registered and used in countries with advanced technology and wide experience with COVID-19,” such as China and Singapore.
FDA Director General Eric Domingo said the rapid test kits will help clear the country’s testing backlog, but noted that PCR-based test kits — used in laboratories to extract genetic material from throat and nose swabs to compare it with the COVID-19 virus — are still more reliable.
The Department of Health (DOH) says PCR-based tests are still the “current gold standard for diagnosing COVID-19.”
Over the weekend, the DOH announced that four other labs will now be able to conduct full-scale COVID-19 testing, including the San Lazaro Hospital in Manila, Vicente Sotto Memorial Medical Center in Cebu City, Southern Philippines Medical Center in Davao City, and Baguio General Hospital in Baguio City.
Prior to this, the country’s sole testing laboratory for COVID-19 was the Research Institute of Tropical Medicine (RITM) in Muntinlupa City, and the sub-national labs had to send their tests to RITM for validation.
According to Health Undersecretary Maria Rosario Vergeire, this is why the four labs had only been able to process up to 160 tests per day, while RITM can process up to 1,000 tests per day.
Vergeire added that the four hospitals have now been fully certified, which “means they no longer have to send their positive cases to the RITM for validation because they’re now certified to do individual testing. They can now release results for those positive for coronavirus.”
Vergeire said that the Lung Center of the Philippines is likewise expected to receive full certification to test for COVID-19 by March 30, while 30 other private and public laboratories across the country are undergoing different stages of certification. Of the 30, seven facilities are in the “advanced stages” of being certified.




Reader Interactions