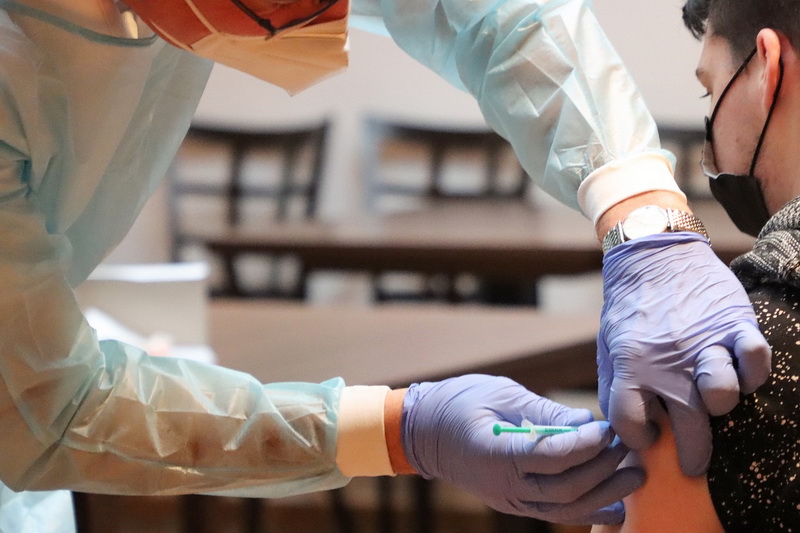
Update Jan. 22: Thailand’s FDA granted emergency approval to the AstraZeneca vaccine, of which 50,000 doses manufactured in Italy will arrive in February followed by another 150,000 by April. At two doses per person, that’s enough to inoculate 0.14% of the population.
A moderately effective vaccine already approved in several major markets may be available for limited use in Thailand sooner than expected.
The AstraZeneca vaccine developed with Oxford University and approved for use in the United Kingdom and United States is expected to win FDA authorization for emergency as early as this week. It could then reach patients as soon as next month, according to a top health official.
The FDA review of the British- and Swedish-developed vaccine’s efficacy and safety is looking good so far, Disease Control Department Director Opas Karnkawinpong said today. The vaccine could be used for the highest-priority cases in February, including medical personnel and people with underlying conditions.
While some regional neighbors have already begun inoculations, Thailand is lagging behind. No vaccines were expected until next month’s arrival of a Chinese-developed vaccine with doubts about its efficacy, followed by produced domestically doses of the AstraZeneca vaccine.
Top FDA official Surachok Tangwiwat said the doses now under consideration have been manufactured by other countries. He did not specify which country was selling unused doses, how many were being imported, or their cost to taxpayers. Authorization is pending some additional documentation, he added. The approval could accelerate manufacture of the vaccine in Thailand by Siam Bioscience, a new pharma player owned by the palace.
The AstraZeneca vaccine has completed trials and been approved for use in several nations with stringent requirements, but at 70% is considerably less effective than some of its competitors.
It is part of an internationally funded effort to develop a vaccine to be made available to all at a low cost. In November, the cabinet set aside THB6 billion (USD$200 million) for the program, which come with the know-how to produce the vaccine at home – by Siam Bioscience.
Thailand expects the first batch of 200,000 doses from China’s Sinovac Biotech in February. Its CoronaVac vaccine has not completed Phase 3 trials and estimates of its efficacy have swung between roughly 50% and 63%. Health officials last week said the vaccine may not win approval until Feb. 14 as the company doesn’t have representatives in Thailand.
Thailand today reported another 171 cases, 158 of which were cases of domestic transmission. Thirteen were found in Bangkok.
Related
Happy Covid-versary! Thailand’s expats at back of line for COVID-19 vaccine
Thailand to vaccinate 33M for free. Here’s what’s coming, and when.