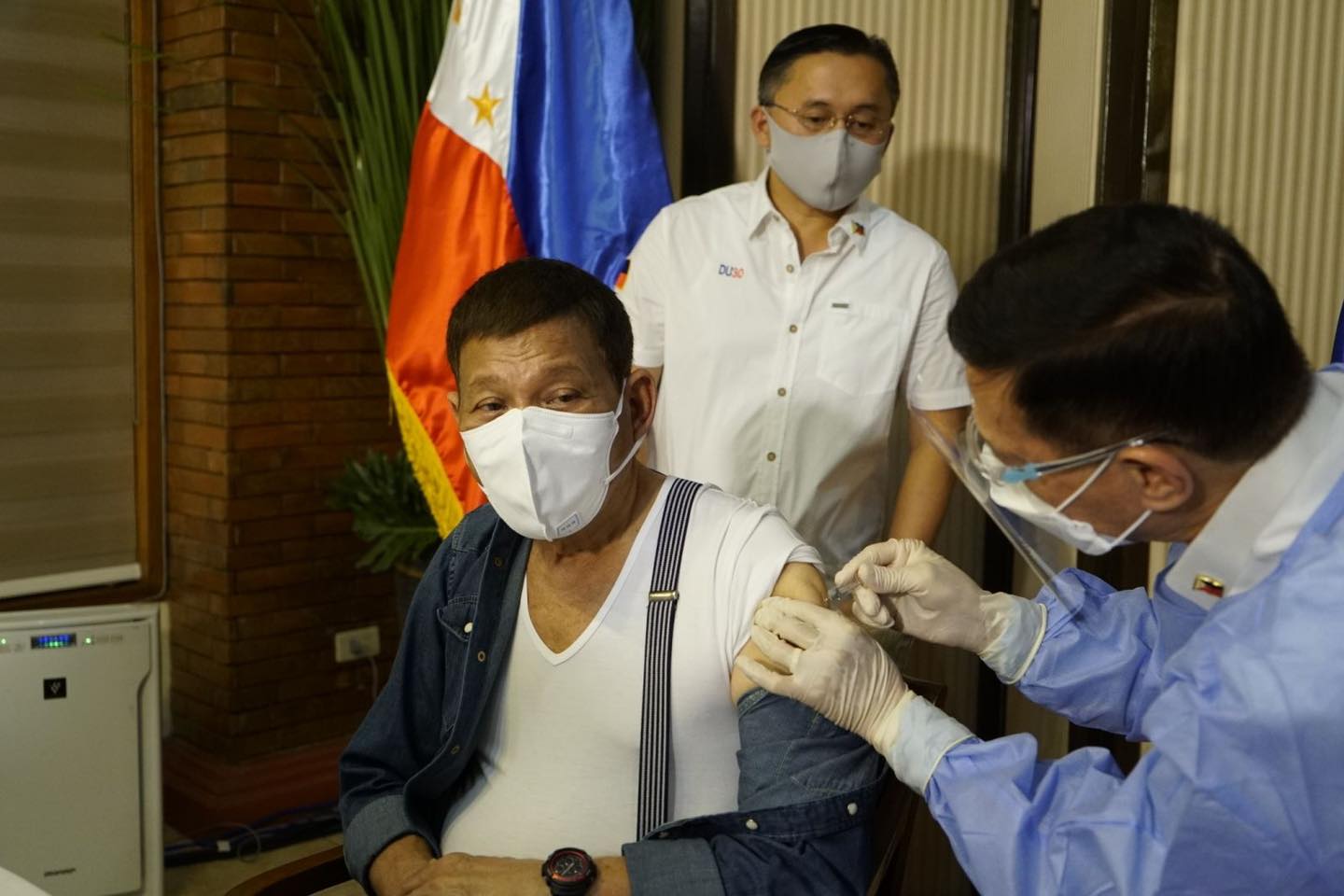
The Philippine Food and Drug Authority (FDA) has yet to authorize the use of Sinopharm, the China-made vaccine that President Rodrigo Duterte received yesterday.
Smuggled doses of Sinopharm were injected on Duterte’s bodyguards in October, but it was only in February that it received a compassionate special permit for it to be used by members of the Presidential Security Group (PSG). Duterte received Sinopharm yesterday, and he was vaccinated by Health Secretary Francisco Duque.
“I think it’s a personal preference. Sinopharm hasn’t been studied by the FDA,” Eric Domingo, the agency’s director-general, said in an interview with Teleradyo.
Read: NCR Plus residents still prohibited from traveling for leisure, says Puyat
“When you say compassionate special permit, that’s not the authorization given by the FDA. In this case, the head of the PSG hospital was the one who guaranteed that he studied the vaccine and they take full responsibility for it. Here at the FDA we haven’t evaluated that vaccine.”
However, he said that the World Health Organization has studied the vaccine, and the organization hasn’t found anything wrong with it yet.
“The vaccine is safe because it uses an inactivated virus like the one from Sinovac. They saw that the efficacy is good,” he said.
“The WHO is continuing with the evaluation for this vaccine. So far it looks good based on the results,” he added.
The Philippines has recorded a total of 1,062,225 COVID cases, including 17,525 deaths.




Reader Interactions